A galvanic cell is constructed using the two hypothetical half-reactions 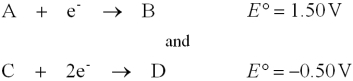
a.Write down the balanced equation representing the cell reaction.
b.Calculate the standard potential of this cell,E°cell.
c.Calculate G° for the cell reaction.
Definitions:
Productivity
The measure of the efficiency of a person, machine, factory, system, etc., in converting inputs into useful outputs.
Labour Hour
A measure of the amount of work performed by a worker in one hour.
Services
Activities, benefits, or satisfactions offered for sale that are essentially intangible and do not result in the ownership of anything.
Goods
Tangible items that are produced, bought, or sold.
Q10: Select the nuclide that completes the following
Q12: A portfolio containing a mix of stocks,bonds,and
Q12: For a gas-phase equilibrium,a change in the
Q14: The masses of a potassium-40 atom,a proton
Q45: Which one of the following equations correctly
Q65: Examine the following half-reactions and select the
Q70: A reaction has an activation energy
Q81: The substance HOBr is considered<br>A)a weak Arrhenius
Q97: If the electrodes of a voltaic cell
Q119: Which of the following is generally NOT