Given that the Activity Series shown below is accurate,then Li(s)+ K+(aq)→ Li+ (aq)+ K(s)is a spontaneous reaction.
Activity Series = 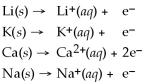
Definitions:
Polar Covalent Bond
A type of chemical bond where a pair of electrons is unequally shared between two atoms.
Water Molecule
A chemical compound consisting of two hydrogen atoms bonded to one oxygen atom, essential for all known forms of life.
Hydrogen Atom
The simplest type of atom, consisting of one proton in the nucleus and one electron orbiting the nucleus, fundamental to the chemistry of life.
Polar Covalent Bond
A type of chemical bond where two atoms share a pair of electrons more or less unequally, creating a molecule with an electrical polarity due to uneven electron distribution.
Q3: What would be the effect on net
Q12: Select the income statement account(s)that would be
Q12: Directors decided to revalue land upwards by
Q13: What is the concentration of H<sup>+</sup> in
Q42: If you prepare a solution by adding
Q45: A sample of salt water will freeze
Q48: What is the effect of the loan
Q49: The driving force that causes electrons to
Q73: Radioactive elements are used for:<br>A)medical diagnosis.<br>B)medical treatment.<br>C)determining
Q105: A saturated solution holds the maximum amount