Tritium ( 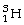
) is an isotope of hydrogen that is sometimes used to make the hands of watches glow in the dark.The half-life of tritium is 12.3 years.After 49 years,approximately how much of the original tritium remains?
Definitions:
Intravenous Solution
A liquid substance administered directly into the vein for therapeutic purposes, including hydration, nutrition, or medication delivery.
Infiltrated
In medical context, it refers to the diffusion or accumulation of foreign substances or cells into tissues, often leading to an inflammatory response.
Chloride
A chemical element or compound (often sodium chloride or potassium chloride) that plays essential roles in fluid balance and acid-base balance in the body.
Anions
Negatively charged ions, which move towards the anode (positive electrode) during electrolysis.
Q5: The most common repeating pattern that is
Q14: A credit balance in retained profits:<br>A) equals
Q20: If Del Ltd reduces the number of
Q43: What is the molecular geometry of the
Q48: What is the [H⁺] in a solution
Q49: The minor component in a solution is
Q70: The control rods of a nuclear reactor:<br>A)regulate
Q74: A gene is a portion of DNA
Q91: What is the balanced oxidation half-reaction
Q100: A solution at 25°C has a hydrogen