Diatomic N2 can react with diatomic H2 to form ammonia (NH3) .The balanced chemical equation is: 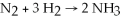
If 6 moles of H2 totally reacted with more than enough N2,how many moles of ammonia would be expected to form?
Definitions:
Future Cash Receipts
Expected cash inflows associated with the future operations of a business, such as revenue from sales or collections on accounts receivable.
Investment Decision
The process of evaluating and selecting from different investment opportunities, often focusing on risk assessment, return expectations, and alignment with overall financial goals.
Return on Investment
A measure used to evaluate the efficiency or profitability of an investment, calculated as the net profit of the investment divided by the cost of the investment.
Accounting Elements
Fundamental components used in financial reporting and analysis, including assets, liabilities, equity, revenues, expenses, and dividends.
Q6: When compounds containing polyatomic ions dissolve,the polyatomic
Q27: Which statement reflects the results of Rutherford's
Q30: An element is discovered that is a
Q43: If 3.011 ×<sup> </sup><br><sup> </sup><br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg"
Q75: What is the name of the compound
Q87: All of the positive charge of an
Q88: Identify the double displacement reactions among the
Q95: Given the chemical equation: 2 Ca +
Q99: The formula of a compound comprised of
Q112: The atomic mass of a single carbon