What is the limiting reactant for the following reaction given we have 2.6 moles of HCl and 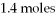
Of Ca(OH) 2?
Reaction: 2HCl + Ca(OH) 2 → 2H2O + CaCl2
Definitions:
Crude Oil
A naturally occurring, unrefined petroleum product composed of hydrocarbon deposits and other organic materials.
Boiling Point
The temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to rapidly vaporize.
Kerosene Components
Kerosene components refer to the mixture of hydrocarbons derived from petroleum, used primarily as fuel in heating and aircraft engines.
Q12: One mole of CO<sub>2</sub> gas contains 1
Q30: Greenhouse gases affect the temperature of the
Q34: Which one of the following is the
Q36: Considering the following precipitation reaction:<br>Pb(NO<sub>3</sub>)<sub>2</sub>(aq)+ 2KI(aq)→ PbI<sub>2</sub>(s)+
Q60: The empirical formula for C<sub>6</sub>H<sub>6</sub> is C<sub>3</sub>H<sub>3</sub>.
Q67: If the electron configuration of a ground
Q72: Suppose a balloon was released from the
Q81: What is the electron geometry if you
Q104: Gas density can be calculated by dividing
Q110: Absolute zero refers to 0°C.