A 24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 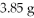
NH3.What is the percent yield for this reaction?
Reaction: N2(g) + 3 H2(g) → 2 NH3(g)
Definitions:
Profitability
A measure of the efficiency and financial performance of a business, indicating the degree to which it generates profit compared to its revenue.
Location Analysis Techniques
A set of methods or models used to evaluate the best geographical location for a business or facility, taking into account factors like cost, accessibility, and market demand.
Factor Rating Method
A location method that instils objectivity into the process of identifying hard-to-evaluate costs.
Service Organizations
Companies that provide intangible products to consumers, such as healthcare, education, and financial services.
Q10: Which substance would be expected to have
Q20: A spectator ion is one that does
Q37: Considering the following precipitation reaction:<br>Pb(NO<sub>3</sub>)<sub>2</sub>(aq)+ 2KI(aq)→ PbI<sub>2</sub>(s)+
Q39: The formula for potassium chlorate is KClO<sub>3</sub>.The
Q53: If the kelvin temperature of a gas
Q60: The empirical formula for C<sub>6</sub>H<sub>6</sub> is C<sub>3</sub>H<sub>3</sub>.
Q63: All ionic compounds dissolve in water.
Q68: A "shielding gas" mixture of argon and
Q70: How many moles of bromine gas are
Q94: What is the theoretical yield of waffles