If the theoretical yield of the reaction below corresponds to 25.3 g and the percent yield of the reaction is known to be reproducibly 81.1%,calculate the actual yield.
Given: 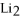
O + 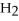
O → 2 LiOH
Definitions:
Equilibrium Quantity
The quantity of goods or services supplied is equal to the quantity of goods or services demanded at the market equilibrium price.
Supply Curve
A graphical representation showing the relationship between the quantity of goods that producers are willing to sell and the price levels of those goods.
Market
A venue where buyers and sellers engage in trade, determining the price of goods and services through the laws of supply and demand.
Equilibrium
A state in economic analysis where supply equals demand, leading to stable prices and quantities in the market.
Q6: What is the charge on the barium
Q11: Gases fill the entire volume of their
Q24: What is the gas produced when hydrochloric
Q28: Chlorine and bromine have very similar chemical
Q35: The empirical formula mass must be 25.0
Q43: When the equation,_O<sub>2</sub> + _C<sub>6</sub> H<sub>14</sub> →
Q48: Metal cations that form more than one
Q87: A chemist wishes to perform the following
Q95: What is the final pressure (expressed in
Q97: Thermal energy flows into the reaction and