What is the limiting reactant for the following reaction given we have 2.6 moles of HCl and 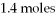
Of Ca(OH) 2?
Reaction: 2HCl + Ca(OH) 2 → 2H2O + CaCl2
Definitions:
Unconscious Thoughts
Mental processes occurring without conscious awareness, influencing behaviors, decisions, and emotions in hidden ways.
Objective Criteria
Standards or benchmarks that are not influenced by personal feelings or opinions and can be measured or evaluated by others independently.
Interjudge Agreement
Interjudge agreement refers to the level of consistency among different judges or observers in their assessments or ratings of a specific phenomenon.
Reaction Formation
A defense mechanism where an individual unconsciously replaces an undesirable or anxiety-inducing emotion or desire with its exact opposite.
Q15: Which of the following statements is false?<br>A)Evaporation
Q20: Which element is represented by the electron
Q32: What is the mass ratio of Na
Q43: As you increase temperature,you increase the average
Q49: When you have 4 electron groups,the electron
Q51: What would be the formula of the
Q75: Substance A is a molecular compound that
Q88: A 5.00 liter balloon of gas at
Q89: The tragedy at Lake Nyos in Cameroon,West
Q96: The reaction CH<sub>4</sub> (g)+ 2 O<sub>2</sub> (g)→