A 24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 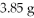
NH3.What is the percent yield for this reaction?
Reaction: N2(g) + 3 H2(g) → 2 NH3(g)
Definitions:
Platinum
A chemical element with the symbol Pt and atomic number 78, a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal.
Atomic Symbol
A one or two-letter notation derived from the Latin name of an element, used to represent an element in chemical equations.
Tin
A chemical element with the symbol Sn and atomic number 50, known for its malleability, ductility, and resistance to corrosion.
Endocrine Disruptors
Chemicals that can interfere with the endocrine (hormone) system in the body, potentially causing developmental, reproductive, neural, and immune effects.
Q3: The ionization energy of lithium is higher
Q10: Lewis structures only use the valence electrons
Q24: A situation where two opposite processes are
Q35: What is the mass number of the
Q60: What is the formula mass for potassium
Q68: How many grams of the excess reactant
Q75: What is the molecular geometry of SiH<sub>4</sub>?<br>A)bent<br>B)linear<br>C)tetrahedral<br>D)trigonal
Q100: The limiting reactant is the product that
Q102: The elements with the highest electronegativity values
Q113: To solve problems using Charles's Law,which mathematical