Which is the excess reactant in the following reaction given that you start with 15.5 g of Na2S and 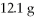
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
Definitions:
Property Taxes
Levies imposed by local governments on real estate properties, calculated based on the value of the property.
Opportunity Costs
Sacrificing potential profits from other possibilities when a certain option is selected.
Equity Capital
Funds raised by a company in exchange for a share of ownership in the company, often through the sale of stock.
Depreciation
The process of allocating the cost of a tangible or physical asset over its useful life, reflecting the decrease in value over time.
Q14: What is the formula mass of sulfurous
Q22: Which sequence below represents the proper order
Q33: A 42.7 gram sample of potassium nitrate
Q43: When the equation,_O<sub>2</sub> + _C<sub>6</sub> H<sub>14</sub> →
Q45: One of the characteristics of an acid-base
Q65: What is the mass of 0.560 moles
Q73: Which is the limiting reactant in the
Q78: One mole of ammonium nitrate contains:<br>A)3 moles
Q84: The Lewis structure for O<sub>2</sub> contains a
Q105: The compound sodium sulfate is soluble in