If the theoretical yield of the reaction below corresponds to 25.3 g and the percent yield of the reaction is known to be reproducibly 81.1%,calculate the actual yield.
Given: 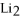
O + 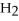
O → 2 LiOH
Definitions:
Altruism
The selfless concern for the well-being of others, often resulting in acts of kindness or generosity without expectation of personal gain.
Happiness
Happiness is an emotional state characterized by feelings of joy, satisfaction, contentment, and fulfillment.
Ingroup Bias
The tendency to favor and extend loyalty to members of one's own group over those of a different group.
Political Party
An organized group that seeks to achieve political power by participating in electoral processes and promoting specific ideologies.
Q5: The double bond is shorter and stronger
Q9: Which color of the visible spectrum has
Q12: One mole of CO<sub>2</sub> gas contains 1
Q12: Increasing the temperature increases the vaporization rate
Q27: Which statement about ozone is TRUE?<br>A)Ozone normally
Q28: How many grams of water are theoretically
Q35: Carbon monoxide contains resonance Lewis structures.
Q87: "Molar Mass" can be calculated using the
Q103: The rate of evaporation will increase if
Q105: Which statement about surface tension is FALSE?<br>A)Liquids