Which of the following diatomic elements would have a mass of 19.08 grams stored in a 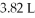
Container at 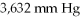
And 100°C? (R= 0.0821 L atm/ mol K)
Definitions:
Exchange Rate
The ratio at which one nation’s currency can be exchanged for another nation’s currency.
National Rate
A standard or average interest rate, unemployment rate, or other statistical measure that applies across an entire country.
Q11: Evaporation is an endothermic process.
Q27: Which of the following compounds is a
Q40: Which of the following is TRUE of
Q43: Consider a 1.0 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="Consider a
Q50: The pH of a 0.0001 M NaOH
Q51: Which compound below is the most soluble
Q53: Bromine has 17 valence electrons.
Q60: Which statement is TRUE in describing what
Q67: The major component in a solution is
Q73: Water has a heat of vaporization of