Which of the following diatomic elements would have a mass of 19.08 grams stored in a 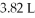
Container at 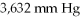
And 100°C? (R= 0.0821 L atm/ mol K)
Definitions:
Signs
In medical or clinical contexts, signs are objective evidence of disease or physical changes in the body, observed by a healthcare professional.
Symptoms
Signs or indications of a disease or condition experienced by an individual.
Away From
A phrase indicating movement or direction leading from a specific point or location.
Middle
Being at a point or position equally distant from the sides, edges, or ends; centrally located.
Q4: Blue light travels at a faster speed
Q13: What is the change in the freezing
Q35: The boiling point is the temperature at
Q52: What is the molecular geometry if you
Q57: What type of a reaction occurs when
Q57: Tap water contains dissolved nitrogen and oxygen.
Q59: Bases feel slippery because they react with
Q70: In order for a substance to be
Q74: The shorter the wavelength of light,the more
Q81: Ion-dipole forces occur in mixtures of ionic