How many joules of heat are needed to completely vaporize 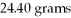
Of water at its boiling point?
Given 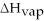
= 40.6 kJ/mol
Definitions:
Patient-Focused Care
A delivery model that brings all services and care providers to the client.
Cross-Trained
Pertaining to individuals who have been trained in multiple disciplines or skill sets, enhancing their ability to perform diverse tasks or roles within an organization.
Quaternary
Pertaining to the fourth level, often used in context with advanced levels of health care that involve highly specialized and complex procedures.
Complex Abdominal
Refers to abdominal conditions or surgeries that are intricate due to the presence of multiple health issues, previous surgeries, or complications.
Q9: A solution with a concentration of 0.000
Q25: How many kJ of heat are needed
Q39: How many kilojoules of heat are needed
Q40: Ion-dipole force is considered the weakest of
Q45: In examining the formula for acetic acid,HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>,the
Q49: Bases have a bitter taste.
Q64: Having eight valence electrons is very stable
Q73: Water has a heat of vaporization of
Q83: What molarity should the stock solution be
Q100: Which compound listed below will dissolve in