How many joules of heat are needed to completely vaporize 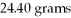
Of water at its boiling point?
Given 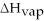
= 40.6 kJ/mol
Definitions:
Budget Constraint
A economic term that represents the limitations on the consumption choices of individuals based on their income and the prices of goods and services.
Tomatoes and Nectarines
Examples of perishable goods often used in discussions about agriculture and consumer choice.
Marginal Utility
The extra satisfaction or utility that an individual gains from consuming an additional unit of a good or service.
Price of Jam
The current market cost at which jam is sold, varying based on factors like quality, brand, and ingredients.
Q2: For the reaction 2 A ⇌ B,the
Q16: In the following reaction,<br>Zn (s)+ CuSO<sub>4</sub> (aq)→
Q34: To achieve the largest battery voltage possible,the
Q42: How many grams of sodium metal are
Q89: Substances that can act both as an
Q100: Which of the following statements is TRUE
Q105: What is the concentration of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg"
Q108: How many eggs are needed to make
Q110: How many subshells are there in the
Q111: Gases typically have high densities in comparison