How many kJ of heat are needed to completely vaporize 3.30 moles of 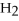
O? The heat of vaporization for water at the boiling point is 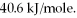
Definitions:
Delivery Times
The duration it takes for goods or services to be delivered from when an order is placed to when it is received by the customer.
Random Sample
A subset of a statistical population where each member has an equal chance of being chosen.
Variability
The measure of how spread out or scattered the points in a data set are.
Average Ages
The mean age of a group of individuals, calculated by summing their ages and dividing by the number of individuals.
Q18: How many grams of the excess reactant
Q21: What is the equivalent pressure of 968
Q34: The ammonium ion contains 10 valence electrons.
Q53: Which statement below is FALSE?<br>A)The rate of
Q58: Which one of the following molecules is
Q72: Salt water is an example of a
Q76: The expected order of density for matter
Q79: In the following reaction: NH<sub>4</sub><sup>+ </sup>(aq)+ H<sub>2</sub>O
Q88: It is important to identify lone pairs
Q110: Which battery system is based on half