Given that the freezing point depression constant for water is 1.86°C kg/mol,calculate the change in freezing point for a 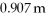
Sugar solution.
Definitions:
Pretreatment Medication
Medication given before a medical procedure or treatment to prevent or minimize potential side effects.
Limb Restraints
Devices or methods employed to restrict the movement or mobility of the limbs, typically used for medical or safety reasons.
Vegetative Signs
Physical symptoms primarily associated with severe depression or other psychiatric conditions, such as changes in sleep, appetite, or energy levels.
Laxatives
Substances taken to stimulate bowel movements or to relieve constipation by softening the stool.
Q12: Which of the following atoms has the
Q22: What is the molarity of a solution
Q32: The strength of the surface tension is
Q61: A sample of salt water will freeze
Q67: What happens to the equilibrium position of
Q84: Which of the following is NOT true?<br>A)The
Q86: The solubility of solids in water generally
Q102: Balance the redox reaction in acid solution:
Q106: The rate of spontaneous nuclear decay:<br>A)can be
Q120: Osmotic pressure is:<br>A)the pressure required to stop