What is the concentration of sodium chloride in the final solution if 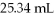
Of 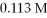
BaC 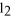
Completely reacts and the total volume of the reaction is 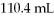
,given the reaction:
Ba 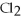
(aq) + N 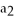
S 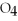
(aq) → BaS 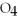
(s) + 2NaCl (aq)
Definitions:
Variable Cost
Costs that vary in direct proportion to changes in a business's production volume or level of activity.
Unit Product Costs
The total cost associated with producing a single unit of product, including direct materials, direct labor, and overhead.
Intermediate Product
A product that requires further processing before it becomes a finished good.
Processed Further
Refers to additional operations or treatments applied to a product after its initial processing stage.
Q13: The Lewis structure,[: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="The Lewis
Q15: From the activity list included in this
Q28: Chlorine and bromine have very similar chemical
Q38: Sodium hydroxide is often used in the
Q47: Which state of matter has a high
Q48: Which state of matter has a high
Q54: Without intermolecular forces,solids and liquids would not
Q89: What is the major component of the
Q92: Which statement is NOT true about "p"
Q120: At STP,12.69 g of a noble gas