Given that the freezing point depression constant for water is 1.86°C kg/mol,calculate the change in freezing point for a 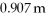
Sugar solution.
Definitions:
Subjective
Based on or influenced by personal feelings, tastes, or opinions rather than external facts or evidence.
Words and Actions
This phrase typically emphasizes the importance of aligning one's spoken words with their actions, often used in contexts discussing integrity, leadership, and personal accountability.
Uniform Electronic Transactions Act
Legislation that establishes the legal validity and enforceability of electronic signatures and records for transactions.
Electronic Contracts
Agreements that are created, transmitted, and signed digitally, utilizing electronic means.
Q13: The Lewis structure,[: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="The Lewis
Q15: Molecules containing carbon always assume a tetrahedral
Q32: A sacrificial electrode works by being reduced
Q34: What is the missing particle? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg"
Q63: Which atomic solid has the highest melting
Q75: Which of the following isotopes contains the
Q80: In a solution that has a pH
Q105: Which statement about surface tension is FALSE?<br>A)Liquids
Q110: Which battery system is based on half
Q121: What is the initial temperature of a