Multiple Choice
For the reaction 2 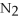
O(g) ⇌ 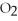
(g) + 2 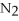
(g) ,what happens to the equilibrium position if the pressure increases?
Definitions:
Related Questions
Q11: An open -chain (noncyclic)hydrocarbon will always have
Q29: Which of the following is not a
Q52: What is the [H⁺] in a solution
Q57: The bases on one strand of DNA
Q63: What are the products from the combustion
Q70: Phosphorous-32 has a half-life of 14.3 days
Q74: In the formation of a secondary protein
Q85: Evaporation and condensation are opposite processes.
Q93: Collisions between reactant molecules do not always
Q110: The minor component in a solution is