Consider the following endothermic equilibrium reaction:
Fe2O3 (s) + 3H2 (g) ⇌ 2Fe (s) + 3 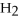
O (g)
Which of the following actions will result in a shift to the left (towards reactants) ?
Definitions:
Deamination
Deamination is the biochemical process that removes an amino group from an amino acid, resulting in the release of ammonia and alteration of the amino acid structure, often taking place in the liver.
Amino Group
An amino group is a functional group consisting of a nitrogen atom attached to two hydrogen atoms (–NH2) and is a fundamental component of amino acids and thus proteins.
Amino Acids
Organic compounds that serve as the building blocks of proteins. They contain an amino group and a carboxylic acid group, and they vary by the side chain attached to their alpha carbon.
Nitrogenous Waste
Waste products containing nitrogen, such as urea, ammonia, and uric acid, produced by the metabolism of proteins and nucleic acids in organisms.
Q9: What must be TRUE for a reaction
Q44: During the first half-life you lose half
Q50: Corrosion can be prevented by all of
Q51: Summarize the main features of the four
Q61: Solids usually have much greater densities than
Q66: Molality is calculated by dividing grams of
Q74: Which of the following structural formulas is
Q91: A compound with a very large <img
Q95: How many isomers exist for the alkane
Q113: Which type of emission has a negative