Given the choice of adding ions of Mg 2+ (aq) ,Ca 2+ (aq) ,Sr 2+ (aq) or Ba 2+ (aq) to a solution of carbonate ions,CO3 2-,which choice would precipitate an insoluble carbonate compound first?
Use the following 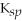
Data:
MgCO3 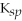
= 6.82 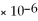
CaCO3 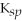
= 4.96 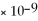
SrCO3 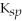
= 9.30 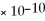
BaCO3 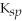
= 5.00 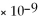
Definitions:
Globalization
The process by which businesses or other organizations develop international influence or start operating on an international scale, leading to increased interconnectedness and interdependence of countries.
Adolescence
The transitional developmental period between childhood and adulthood, characterized by significant physical, psychological, and social changes.
Moral Reasoning
The process of determining right or wrong in a given situation based on ethical principles.
Piaget
Swiss psychologist known for his pioneering work in child development and cognitive theory, emphasizing how children acquire knowledge and understanding.
Q4: The solubility of Pb(NO<sub>3</sub>)<sub>2</sub> is 55 grams
Q50: Lipids include all of the following compounds
Q51: Summarize the main features of the four
Q57: Alkynes are compounds with at least one
Q70: Avogadro's Law is expressed as:<br>A)V is proportional
Q70: What are the principal forces holding ice
Q103: The exact shape that a protein takes
Q105: A solution that is 13.58 percent by
Q112: Liquids that are viscous flow more slowly
Q116: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="For the