Calculate the equilibrium constant for the reaction below given that 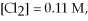
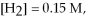
And 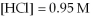
At equilibrium.
Given: 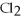
(g) + 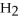
(g) ⇌ 2HCl(g)
Definitions:
Nitrification
Conversion of ammonium to nitrate.
Trophic Category
A classification describing the position of organisms within a food chain, based on their feeding relationships and energy transfer.
Detritivore
An organism that feeds on dead organic matter, largely contributing to decomposition and nutrient recycling in ecosystems.
Earthworm
Earthworms are tube-shaped, segmented worms found in soil that play a crucial role in aerating soil and breaking down dead organic matter.
Q5: The only difference between starch and cellulose
Q55: Radioactive elements are used for:<br>A)medical diagnosis.<br>B)medical treatment.<br>C)determining
Q84: It is expected that the Human Genome
Q85: What is the mass percent of a
Q90: What are the products of a neutralization
Q90: A globular shape is an example of
Q94: Intermolecular forces hold atoms and molecules in
Q105: Which statement about surface tension is FALSE?<br>A)Liquids
Q106: The rate of spontaneous nuclear decay:<br>A)can be
Q116: isobutyl<br>A)-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>B)R- OH<br>C)-CH<sub>2 </sub>- CH<sub>2 </sub>- CH<sub>3</sub><br>D)- CH<sub>3</sub><br>E)-