Consider the Following Reaction 3Fe(s) + 2Al3+(aq)
This Reaction Takes Place in the Electrochemical
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below. 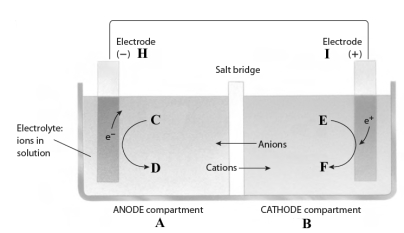
Complete the following questions using the letters shown in the image.
-If the electrolyte contains nitrate ions, they will move from compartment ___ to compartment ___.
Definitions:
Slimy Mucus
A thick, slippery secretion produced by mucus membranes to protect and lubricate surfaces.
Hagfish
Jawless marine fish known for their ability to produce a large amount of slime or mucus when disturbed.
Swim Bladder
Of many ray-finned fishes, a gas-filled organ that adjusts buoyancy.
Buoyancy
The upward force that supports objects in fluid, preventing them from sinking.
Q3: The fuel identified as M85 is<br>A)
Q11: Which does not contain a nitrogen atom
Q12: The fusion of nuclei requires<br>A) a critical
Q23: Biochemical oxygen demand (BOD) is<br>A) high for
Q39: A new element has the symbol Y
Q39: Where do sulfur dioxide and sulfuric acid
Q39: Rubbing alcohol is<br>A) methanol, CH<sub>3</sub>OH.<br>B) 2-propanol, CH<sub>3</sub>CHOHCH<sub>3</sub>.<br>C)
Q63: How many electrons in the valence shell
Q72: Which substance has ionic bonds between atoms?<br>A)
Q86: Which of the following pairs of atoms