Consider the Following Reaction 3Fe(s) + 2Al3+(aq)
This Reaction Takes Place in the Electrochemical
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below. 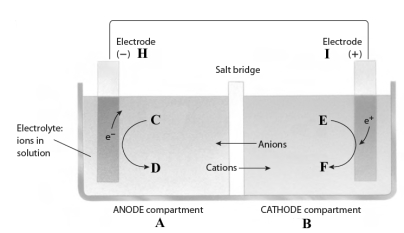
Complete the following questions using the letters shown in the image.
-If the electrolyte contains nitrate ions, they will move from compartment ___ to compartment ___.
Definitions:
Small Business
A business that is privately owned and operated, with a small number of employees and relatively low volume of sales.
Expenses
Costs incurred by a business in the process of earning revenue, such as rent, salaries, and utilities, which are used to assess company profitability.
Revenue
The income generated from normal business operations, usually from the sale of goods and services.
Score Norming
A process in assessment and education to adjust scores on test forms that might be easier or harder than a standard, ensuring fairness and comparability across different test administrations.
Q1: Which will have the largest molar mass?<br>A)
Q4: Anabolic steroids would be classified as _.
Q5: Substances with solubility properties similar to those
Q13: Which class of food nutrient is not
Q18: Which of the following is a free
Q20: Consider the following two statements.<br>"Energy can be
Q22: The density of copper is 8.96 g/mL
Q35: In the following set, which atom is
Q46: Which fuel has the largest heat of
Q66: Which is not characteristic of liquids?<br>A) freezing