Consider the following model of ethanol, CH3CH2OH. 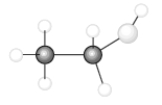 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-Using the data in the following table, 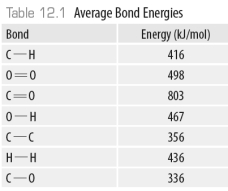 The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
Definitions:
Interest Level
The degree of curiosity or concern one has in a subject or activity.
Teamwork
The cooperative effort by the members of a group to achieve a common goal.
Directive Style
A leadership approach characterized by providing clear, explicit instructions and expectations while closely supervising tasks.
Formal Structure
Organized and systematic arrangement of relationships, duties, and roles within an organization.
Q6: The reaction that is identical to a
Q7: Identify the pair that are allotropes.<br>A) pencil
Q20: Which atom exists as a diatomic molecule
Q32: Consider the following image. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4139/.jpg" alt="Consider
Q35: The chloralkali process uses electrolysis of aqueous
Q35: Which of the following is not true
Q48: What is conserved when one balances a
Q49: Precipitation with a pH of 8.0 would
Q52: Which substance named below is not an
Q84: What is the molecular geometry of carbon