Consider the following model of ethanol, CH3CH2OH. 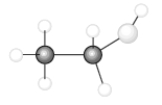 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-Using the data in the following table, 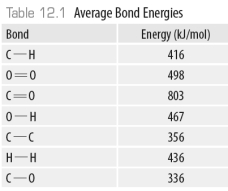 The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
Definitions:
Idle Capacity
Unused or underutilized resources within a business, often indicating inefficiency, where machinery, space, or labor is not being employed to full capacity.
Variable Overhead Cost
Costs that vary with the level of production output, such as supplies and utilities for manufacture.
Variable Costing
An accounting method that includes only variable production costs (direct materials, direct labor, and variable manufacturing overhead) in the cost of goods sold, excluding fixed overhead costs.
Absorption Costing
A cost accounting method that includes all manufacturing costs - direct materials, direct labor, and both variable and fixed overhead - as part of the cost of a finished product.
Q5: Fractional distillation of petroleum<br>A) separates and purifies
Q5: The Flavr Savr tomato is an early
Q18: The main source of the metal magnesium,
Q18: Consider the following Lewis dot structure for
Q22: Glass is annealed so that<br>A) silicon is
Q23: What produces the tremendous energy of a
Q38: The following energy profile represents an<br>exergonic reaction.
Q40: How many grams are in 2.40 mol
Q44: Which polymer material occurs naturally?<br>A) nylon<br>B) Dacron<br>C)
Q53: Which is not true about carbohydrates?<br>A) can