Calculate 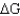 for a reaction given
for a reaction given 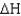 = 15.4 kJ/mole and
= 15.4 kJ/mole and 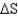
= 2.0 J/K at 298 K.Is this reaction spontaneous in the forward direction?
Definitions:
Manifest Needs
Learned or acquired needs that are easily perceived.
High Achievers
Individuals who exhibit a consistent and exceptional level of success in their endeavors, often driven by personal standards of excellence.
Prefer to Work Alone
A personal or situational preference for completing tasks or projects independently, without collaboration.
Plan For Success
A comprehensive strategy or approach intended to achieve specific goals or outcomes, ensuring positive results.
Q14: Which of the following statements is true
Q18: Phospholamban contains two phosphorylation sites,Ser16 and Thr17.Compare
Q31: Triacylglycerols are neutral molecules made of<br>A) three
Q43: When cells isolated from the spleen are
Q61: The amino acid with the most hydrophobic
Q63: If you were a biochemist who just
Q65: The amino acid with the neutral side
Q70: Differentiate between germ-line cell mutations and somatic
Q88: A newly discovered transporter protein is being
Q104: Time lag is one characteristic used to