Calculate 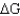 for a reaction given
for a reaction given 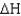 = 15.4 kJ/mole and
= 15.4 kJ/mole and 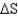
= 2.0 J/K at 298 K.Is this reaction spontaneous in the forward direction?
Definitions:
Uniquely Treated Paper
Paper that has undergone special processing or treatment to give it distinct characteristics or functions.
Paid in Full
A term indicating that a bill or a debt has been completely paid off and no amount remains due.
Full Balance
This term could refer to the complete settling of a financial account, leaving no outstanding balance.
Endorsement
Official approval or support, often used in the context of public approval by a prominent figure or certification by a regulatory authority.
Q31: A clerk reorders 250 items when the
Q40: Explain the differences among the three types
Q45: What do Ramachandran plots show?<br>A) Only some
Q45: Which of the following is NOT an
Q50: Enzymes and chromosome are found where in
Q55: Of the 20 common amino acids,how many
Q74: Compare the structure of a phospholipid bilayer
Q84: In solid-phase peptide synthesis,which reagent is used
Q89: What are the three primary functions performed
Q111: Data flow diagrams represent the physical system.