Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 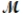 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 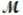 = 32.00 g/mol) is allowed to react. What mass of aluminum oxide (
= 32.00 g/mol) is allowed to react. What mass of aluminum oxide ( 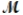 = 101.96 g/mol) can be formed?
= 101.96 g/mol) can be formed?
Definitions:
Net Liability Balance
The amount by which liabilities exceed assets, showing a negative balance sheet position.
Foreign Currency
Currency used in a country other than one's own, impacting transactions and financial reporting in international business operations.
Translation Adjustment
Translation adjustment is an accounting process used to convert the financial statements of a company's foreign operations into the company's functional currency, accounting for exchange rate changes.
Exchange Rate
The rate at which one currency can be exchanged for another, typically used in international trading and travel.
Q20: A single atom of hydrogen has a
Q20: The most significant contribution to modern science
Q35: Real estate agencies can be created several
Q53: A column of the periodic table is
Q60: If a court proceeds to confirm a
Q61: When a wooden match burns in air,
Q66: Which measurement is expressed to 4 significant
Q85: The colorless substance, MgF<sub>2</sub>, is used in
Q96: Calcium chloride is used to melt ice
Q101: The substance, KClO<sub>3</sub>, is a strong oxidizer