Tetraphosphorus hexaoxide ( 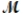 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
Definitions:
Liberal Position
A political ideology favoring more government intervention in economic matters and more social freedoms, emphasizing equality and social justice.
Charles Murray
An American political scientist and author known for his controversial works on intelligence, socioeconomic status, and public policy.
Social And Economic Change
Transformations within a society that affect its economic systems, institutions, social structures, and cultural values over time.
Heritage Of Slavery
The social, economic, and cultural legacies and impacts stemming from the history of slavery in a society.
Q14: a. Calculate the wavelength in nm of
Q18: Title held as "Elva L. Vargas, an
Q31: State Hund's rule, and show how it
Q38: Deed restrictions that apply to all lots
Q55: How many grams of sodium fluoride (used
Q59: Which of the following correctly shows how
Q61: Select the diamagnetic ion.<br>A) Cu<sup>2+</sup><br>B) Ni<sup>2+</sup><br>C) Cr<sup>3+</sup><br>D)
Q82: Calculate the molarity of a 23.55-mL solution
Q83: Phosphorus pentachloride, PCl<sub>5</sub>, a white solid that
Q95: Briefly state the conditions corresponding to STP