What is the percent yield for the reaction PCl3(g) + Cl2(g) PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 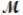 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
Definitions:
Internet Tool
A software or online resource designed to perform or facilitate specific tasks on the internet, such as communication, research, or content creation.
Anonymity
The condition of being anonymous, where an individual's identity is not known or disclosed.
Discrimination
Unfair treatment of individuals or groups based on characteristics such as race, gender, age, or religion.
Minority Groups
Segments of a population that are differentiated from the majority through ethnic, racial, religious, or cultural identity and often experience disadvantage or discrimination.
Q5: A sample of the inert gas krypton
Q21: When a non-occupying investor and an owner-occupant
Q22: The size of an atomic orbital is
Q23: Don and June Seaman, father and daughter,
Q26: The substance, CoCl<sub>2</sub>, is useful as a
Q38: Covalently bonded substances do not necessarily exist
Q49: An evacuated 276 mL glass bulb weighs
Q50: The number 6.0448, rounded to 3 decimal
Q92: What is the name of IF<sub>7</sub>?<br>A) iodine
Q102: Vinegar is a solution of acetic acid,