Tetraphosphorus hexaoxide ( 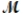 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
Definitions:
Contribution Margin Ratio
This ratio measures how much of a company's revenues will contribute to covering its fixed costs and generating profit, after variable costs have been paid.
Operating Income
Profit generated from normal business operations, calculated as sales minus cost of goods sold and operating expenses.
Fixed Costs
Expenses that do not change in proportion to the activity of a business, such as rent or salaries.
Unit Contribution Margin
The difference between the selling price per unit and the variable cost per unit, used to determine how sales affect profitability.
Q5: Zoning regulations have the greatest impact on
Q16: Which of the following electron configurations is
Q16: Calculate the number of oxygen atoms in
Q18: Select the element whose Lewis symbol is
Q24: For each of the following species, write
Q30: An easement can be terminated in several
Q47: Balance the following equation: <br>B<sub>2</sub>O<sub>3</sub>(s) + HF(l)
Q50: Which one of the following sets of
Q60: If a court proceeds to confirm a
Q99: Covalent compounds, dissolved in water, never produce