What is the percent yield for the reaction PCl3(g) + Cl2(g) PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 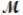 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
Definitions:
Concise Language
The use of few words to convey much information or meaning, avoiding unnecessary details or filler words.
Cardiovascular Danger
A term referring to the risk factors that can lead to diseases affecting the heart and blood vessels.
Blood Pressure
The pressure of circulating blood on the walls of blood vessels, often measured for diagnosis since it is closely related to the force and rate of the heartbeat.
Consistency
The quality of achieving a level of performance which does not vary greatly in quality over time.
Q10: The compound, BaO, absorbs water and carbon
Q10: A homestead is considered terminated when:<br>A) the
Q11: The highest form of ownership a person
Q15: What is the name of the acid
Q16: Which of the following electron configurations is
Q38: Calculate the molar mass of Ca(BO<sub>2</sub>)<sub>2</sub>·6H<sub>2</sub>O.<br>A) 273.87
Q44: Silver chloride is used in photographic emulsions.
Q50: Which of the following fourth-period elements has
Q54: A flask containing argon gas is connected
Q75: Select the correct electron configuration for sulfur