How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 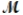 = 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
Definitions:
Ligaments
Tough, fibrous connective tissues that connect bones to other bones, providing joint stability.
Fibrous Bands
Connective tissue structures composed of collagen that commonly link or support organs, often involved in scarring or adhesions.
Range-of-Joint Movement
The full motion range or flexibility of a joint, from fully bent to fully extended.
Center
In healthcare, it often denotes a focal point or hub for specialized treatment and research.
Q3: A judgment is considered to be what
Q9: Electromagnetic radiation of 500 nm wavelength lies
Q25: Magnesium metal (0.100 mol) and a volume
Q37: What is the molecular shape of ClF<sub>2</sub><sup>-</sup>
Q38: Select the precipitate that forms when the
Q46: In covalent bond formation, the potential energy
Q63: The rate of diffusion of a gas
Q65: Ammonium sulfate, (NH<sub>4</sub>)<sub>2</sub>SO<sub>4</sub>, is a fertilizer widely
Q77: All acid-base reactions produce a salt and
Q95: The best Lewis structure for sulfuric acid