How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 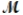 = 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
Definitions:
Prevalence
The proportion of a population found to have a particular condition or characteristic at a specific time.
Depression
A common and serious mood disorder characterized by persistent feelings of sadness, emptiness, or hopelessness, often accompanied by physical symptoms.
Korsakoff's Syndrome
A chronic memory disorder caused by severe deficiency of thiamine, most often associated with alcohol abuse.
Anorexia Nervosa
A serious mental health disorder characterized by an intense fear of gaining weight and a distorted body image, leading to excessive weight loss and inadequate food intake.
Q3: In not more than three sentences, describe
Q3: Calculate the pressure of a helium sample
Q5: Select the arrangement of electromagnetic radiation which
Q12: Select the correct electron configuration for Te
Q15: Select the answer that expresses the result
Q17: State Boyle's Law and illustrate it with
Q19: Which of the following abbreviations of the
Q48: Which one of the following groups does
Q103: If the molecular mass of a gas
Q109: The pressure of sulfur dioxide in a