In the following Lewis structure for ClO3F, chlorine has a formal charge of ____ and an oxidation number of ____. 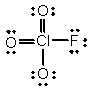
Definitions:
Seceding
The act of formally withdrawing from an organization, union, or especially a federation of states, such as the Southern states seceding from the Union leading to the Civil War.
James Buchanan
The 15th president of the United States, serving immediately prior to the American Civil War.
Political Experience
Refers to the practical experience acquired through involvement or participation in political processes, often considered valuable in governance roles.
Mexican War
A conflict between the United States and Mexico from 1846 to 1848, resulting in the U.S. acquiring territories that include present-day California, Arizona, and New Mexico.
Q23: Consider the reaction: <br>3Co<sup>2+</sup>(aq) + 6NO<sub>3</sub><sup>-</sup>(aq) +
Q28: Select the element with the least metallic
Q38: Covalently bonded substances do not necessarily exist
Q52: The elements from Groups 1A(1) and 2A(2)
Q56: When two atoms form a covalently-bonded diatomic
Q66: In not more than three lines for
Q72: Which of the lines on the figure
Q77: "The pressure of an ideal gas is
Q95: The vapor pressure of pure acetone (propanone)
Q99: Covalent compounds, dissolved in water, never produce