Multiple Choice
The formal charge on Cl in the structure shown for the perchlorate ion is 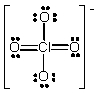
Definitions:
Related Questions
Q6: Select the compound with the lowest (i.e.,
Q8: As a measure of the strength of
Q14: The lattice energy of MgCl<sub>2</sub> is the
Q19: The energy of a hydrogen bond is
Q33: Carbon uses _ hybrid orbitals in ClCN.<br>A)
Q43: In the following reaction, what ions, if
Q61: Select the diamagnetic ion.<br>A) Cu<sup>2+</sup><br>B) Ni<sup>2+</sup><br>C) Cr<sup>3+</sup><br>D)
Q62: Select the classification for the following reaction.
Q103: If the molecular mass of a gas
Q110: The most basic oxides contain elements from