The Lewis structure of formaldehyde, CH2O, is shown. Use VSEPR theory to predict the molecular geometry and the H-C-H bond angle. Outline your reasoning. 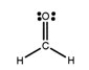
Definitions:
Mass Number
The total number of protons and neutrons in an atom's nucleus, determining the atom's mass.
Protons
Positively charged particles found within an atom's nucleus, contributing to the atom's mass and charge.
Neutrons
Subatomic particles found in the nucleus of an atom, with no electric charge and a mass slightly larger than that of a proton.
X-rays
Electromagnetic waves with wavelengths shorter than those of visible light, capable of penetrating solids and ionizing gases, widely used in medicine and industry for imaging and diagnostic purposes.
Q1: Predict the actual bond angles in SF<sub>3</sub><sup>+</sup>
Q6: Select the compound with the lowest (i.e.,
Q15: Which of the following contains covalent bonds?<br>A)
Q25: Select the correct name for the following
Q36: Two aqueous solutions are prepared: 2.0 m
Q57: Select the classification for the following reaction.
Q57: Which of the following pairs is arranged
Q59: Write balanced equations, showing all reactants and
Q96: Most of the alkali metal salts are
Q110: What word best describes the type of