Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 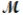 urea = 60.06 g/mol
urea = 60.06 g/mol
Definitions:
Negotiable Instrument
A written promise or order to pay a fixed amount of money, easily transferable from one party to another.
Promissory Note
A written promise to pay a specified sum of money to a certain individual or entity under agreed-upon terms.
Fraudulent Payment
A transaction made with the intent to deceive, defraud, or mislead another party, typically involving the transfer of funds.
Online Transaction Systems
Digital platforms and methods used for conducting financial transactions over the internet.
Q20: The active ingredient in an over the
Q29: Elements with _ first ionization energies and
Q34: The electrostatic energy of two charged particles
Q43: Select the strongest bond in the following
Q48: The difference in energies between the 1s
Q59: Select the correct name for the following
Q64: Which of the following electron configurations represents
Q73: A solution of ethanol (C<sub>2</sub>H<sub>6</sub>O) in water
Q78: Potassium nitrate is a strong reducing agent.
Q81: What types of forces exist between molecules