Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 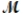 = 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
= 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
Definitions:
Market Risk
The possibility of an investor experiencing losses due to factors that affect the overall performance of the financial markets.
Diversification
A risk management strategy that mixes a wide variety of investments within a portfolio to minimize the impact of any single asset's performance on the overall portfolio returns.
Rule Of 70
A quick formula used to estimate the number of years required for an investment or population to double, calculated by dividing 70 by the annual growth rate.
Interest Rate
The cost of borrowing money or the return earned on investments, typically expressed as a percentage of the principal amount.
Q2: a. What simple experiment could you perform
Q2: The substance Ba(OH)<sub>2 </sub>is considered<br>A) a weak
Q10: Which of the following atoms can expand
Q17: Hybrid orbitals of the sp<sup>3</sup>d type occur
Q19: In which one of the following is
Q28: Silicon carbide is an important industrial chemical.
Q53: Select the correct type for the following
Q58: According to VSEPR theory, a molecule with
Q71: The melting points of metals are only
Q80: According to VSEPR theory, a molecule with