Carbon tetrachloride, once widely used in fire extinguishers and as a dry cleaning fluid, has been found to cause liver damage to those exposed to its vapors over long periods of time. What is the boiling point of a solution prepared by dissolving 375 g of sulfur (S8, 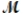 = 256.5 g/mol) in 1250 g of CCl4? Kb = 5.05°C/m, boiling point of pure CCl4 = 76.7°C?
= 256.5 g/mol) in 1250 g of CCl4? Kb = 5.05°C/m, boiling point of pure CCl4 = 76.7°C?
Definitions:
Q15: Which of the following contains covalent bonds?<br>A)
Q50: What is the molecular shape of the
Q52: The following reaction is at equilibrium at
Q55: Assuming that atoms are spherical, calculate the
Q59: Which of the following elements is the
Q85: Select the correct electron configuration for Cu
Q88: Elements of group 1A have higher melting
Q90: Calculate the molarity of a solution prepared
Q100: The equilibrium constant K<sub>c</sub> for the reaction
Q109: Which element forms compounds which are involved