Hexachlorophene is used as a disinfectant in germicidal soaps. What mass of hexachlorophene ( 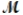 = 406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C? Kb = 3.63°C/m, boiling point of pure chloroform = 61.70°C
= 406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C? Kb = 3.63°C/m, boiling point of pure chloroform = 61.70°C
Definitions:
Option Payoffs
The potential return or outcomes from holding or exercising an options contract.
Q3: In the valence bond treatment, overlap of
Q5: A single covalent bond consists of a
Q19: Valence bond theory is able to explain
Q31: The gas-phase reaction CH<sub>3</sub>NC <font face="symbol"></font> CH<sub>3</sub>CN
Q35: Tetrafluoroethylene, C<sub>2</sub>F<sub>4</sub>, can be converted to octafluorocyclobutane
Q38: a. Draw and name three molecular shapes
Q40: Dinitrogen tetraoxide, N<sub>2</sub>O<sub>4</sub>, decomposes to nitrogen dioxide,
Q51: Which of the following elements has the
Q73: Hydrogenation of double and triple bonds is
Q85: List the three common classes of liquid