Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 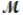 = 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
= 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
Definitions:
Markup Percentage
A percentage increase on the buying price of items intended to account for operational expenses and ensure earnings.
Selling and Administrative Costs
Expenses related to the selling of products or services and the general administrative activities of a business.
Markup Percentage
The proportion added onto the purchase price of goods to accommodate for overhead costs and gain profit.
Product Cost Method
An accounting technique that assigns all costs associated with production to the products, including materials, labor, and overhead.
Q3: A sample of octane in equilibrium with
Q18: Select the element with the highest first
Q19: Which of the following elements is paramagnetic?<br>A)
Q37: Sulfur hexafluoride is a pollutant responsible for
Q38: Covalently bonded substances do not necessarily exist
Q40: Which of the following aqueous liquids will
Q51: Dacron is a _ compound and is
Q54: Amygdalin (Laetrile) was once touted for its
Q82: Name and outline the concept which is
Q90: Consider the oxides XO<sub>2</sub>, where X is