Multiple Choice
Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 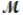 = 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
= 74.55 g/mol) in 100.0 g of water. Assume ideal behavior for the solution; Kf = 1.86°C/m.
Definitions:
Related Questions
Q18: Which of the following is true about
Q33: The elementary reaction HBr(g) + Br(g) <font
Q39: Select the element whose Lewis symbol is
Q48: Predict the ideal bond angles around carbon
Q48: If the solid form of a pure
Q52: Which of the following sets of conditions
Q80: How many valence electrons are there in
Q91: Which, if any, of the following generalized
Q97: Select the correct name for the following
Q112: Which of the following pure substances will