Cyclobutane decomposes to ethene in a first-order reaction. From measurements of the rate constant (k) at various absolute temperatures (T), the accompanying Arrhenius plot was obtained (ln k versus 1/T). 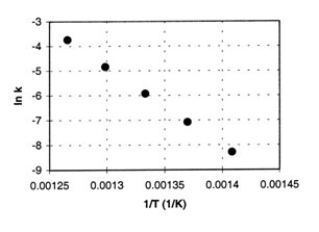 a. Calculate the energy of activation, Ea.
a. Calculate the energy of activation, Ea.
b. Determine the value of the rate constant at 740. K. (In the plot, the units of k are s-1.)
Definitions:
Rate of Return
The increase or decrease in value of an investment over a certain time frame, shown as a percentage of the investment's original price.
Total Assets
The total value of everything a company owns, encompassing cash, stocks, real estate, and machinery.
Common Stockholders
Investors who own shares of common stock in a company, granting them voting rights and a share in the company's profits through dividends.
Operating Expenses
Costs associated with the day-to-day operations of a business, excluding costs directly linked to the production of goods or services.
Q20: The substance H<sub>2</sub>SO<sub>3 </sub>is considered<br>A) a weak
Q33: One characteristic of the monomers that form
Q40: The substance (CH<sub>3</sub>CH<sub>2</sub>)<sub>2</sub>NH is considered<br>A) a weak
Q45: Which relationship best describes <font face="symbol"></font>S° for
Q48: What is the E°<sub>cell</sub> for the cell
Q59: Write balanced equations, showing all reactants and
Q63: Write balanced equations, showing all reactants and
Q67: Which relationship or statement best describes <font
Q67: Select the pair of substances in which
Q72: Predict the ideal van't Hoff factor (i)