Multiple Choice
Consider the following two equilibria and their respective equilibrium constants: (1) NO(g) + 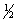 O2(g)
O2(g)  NO2(g)
NO2(g)
(2) 2NO2(g)  2NO(g) + O2(g)
2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
Definitions:
Related Questions
Q5: Compare one mole of ice with one
Q16: For some gas-phase reactions, K<sub>p</sub> = K<sub>c</sub>.
Q27: Which of the following has an effect
Q44: If the activation energy of a reaction
Q48: Consider the figure which shows <font face="symbol"></font>G°
Q57: Hydrogen iodide, HI, is formed in an
Q61: A solution is prepared by adding 100
Q71: The reaction of nitric oxide to form
Q88: Consider the following mechanism for the oxidation
Q98: A 0.100 m K<sub>2</sub>SO<sub>4 </sub>solution has a