Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 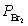 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
Definitions:
Constant Growth DCF Model
A method of valuing a company using the theory that its stock is worth the sum of all its future cash flows, discounted back to their present value at a constant growth rate.
Perpetual Preferred Stock
A type of preferred stock with no fixed maturity date, where dividends must be paid by the company before dividends are paid to common stockholders.
Expected Return
The weighted average of all possible returns for an investment, with weights representing the probabilities of each outcome.
Required Return
The minimum gain investors expect from an investment, considering its risk level; synonymous with required rate of return.
Q12: Two cells are connected in series, so
Q28: Select the correct name for the following
Q29: The polymers containing silicon differ from polymers
Q30: Consider the following redox equation Mn(OH)<sub>2</sub>(s) +
Q38: An increase in temperature increases the reaction
Q65: Examine the following half-reactions and select the
Q73: Testosterone is a male hormone. Identify the
Q84: Hydrogen sulfide will react with water as
Q87: If a solute dissolves in an endothermic
Q99: Arrhenius bases raise the hydroxide ion concentration