The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature. Br2(g) + Cl2(g)  2BrCl(g)
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g) 
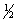 Br2(g) +
Br2(g) + 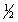 Cl2(g)
Cl2(g)
Definitions:
Psychoanalysis
A theory of human personality development formulated by Freud, based on assertions about unconscious conflict and early psychosexual development; also the method of therapy that draws heavily on this theory.
Unconditional Positive Regard
A term used in psychology to describe a practice where one shows complete support and acceptance of a person regardless of what that person says or does.
Empathic Understanding
The capacity to recognize, comprehend, and directly experience the emotions of another person.
Self-Acceptance
The recognition and acceptance of one's own strengths and weaknesses, leading to a positive self-concept.
Q4: Which relationship best describes <font face="symbol"></font>S° for
Q10: Nitric acid, HNO<sub>3</sub><br>A) is a strong reducing
Q14: Calculate <font face="symbol"></font>S° for the reaction <img
Q45: The millipede ejects the compound shown below
Q54: According to the collision theory of reaction
Q67: All Lewis acids contain at least one
Q79: Select the pair of substances in which
Q99: Arrhenius bases raise the hydroxide ion concentration
Q103: When 14.7 mL of aqueous HBr (a
Q111: Salts of group 1A metals are generally