Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 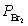 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
Definitions:
Amine
Organic compounds derived from ammonia by replacement of one or more hydrogen atoms by hydrocarbon or other radicals; they play essential roles in biology as building blocks of proteins and neurotransmitters.
Developmental Psychologist
A professional who studies the growth and development of the human mind and behavior across the lifespan.
Newborns
Refer to infants from birth to about two months of age, during which they undergo rapid physical and cognitive development.
Three-Dimensional Patterns
Structured arrangements or designs that have depth, height, and width, giving them a sense of volume or space.
Q6: Calculate the vapor pressure of a solution
Q13: The half-life of a first-order reaction does
Q27: A voltaic cell has a standard cell
Q39: Identify the organic product for the reaction
Q55: When a catalyst is added to a
Q57: Hydrogen iodide, HI, is formed in an
Q87: Changing the amount of a solid reactant
Q88: Which of the following aqueous solutions should
Q92: Consider the equilibrium reaction: N<sub>2</sub>O<sub>4</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5833/.jpg"
Q103: When a chemical system is at equilibrium,<br>A)